Electrochem. Commun., 2016, 73, 63-66 Robert A. Green, Richard C.D. Brown, Derek Pletcher, Bashir Harji
An Extended Channel Length Microflow Electrolysis Cell for Convenient Laboratory Synthesis
A spiral, extended channel length microflow cell designed for routine and convenient application in an organic synthesis laboratory is described. The performance of the cell is demonstrated using two syntheses and it is shown that high selectivities and high conversions in a single pass can be achieved as well as the formation of products at a rate of up to 25 mmol/h (~3 g/h). The cell is also well suited to carrying out the optimisation of reaction conditions with electrolyses completed on a timescale of minutes.
Read More
J. Flow Chem. 2016, 6, 191-197 Robert A. Green, Richard C.D. Brown, Derek Pletcher

Electrosynthesis in Extended Channel Length Microfluidic Electrolysis Cells
In recent papers, laboratory microfluidic electrolysis cells with extended channel lengths (0.7–2 m) and narrow interelectrode gap (≤0.5 mm) have been introduced; these cells permit high conversions at a flow rate consistent with the synthesis of products at a rate of multigrams/hour. Such microflow electrolysis cells must be operated with appropriate control parameters if good performance is to be achieved; thus, this paper emphasizes the correct selection of cell current, flow rate, and counter electrode chemistry. It is also shown that, within the limitations, the cells can be used for a number of electrosyntheses in the synthetic laboratory.
Read More
Org. Lett. 2016, 18, 1198-1201 Robert A. Green, Derek Pletcher, Stuart G. Leach, Richard C. D. Brown
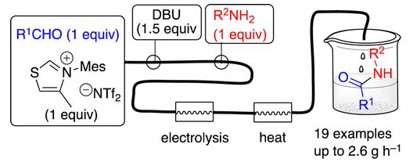
N‑Heterocyclic Carbene-Mediated Microfluidic Oxidative Electrosynthesis of Amides from Aldehydes
A flow process for N-Heterocyclic Carbene (NHC)- mediated anodic oxidative amidation of aldehydes is described, employing an undivided microfluidic electrolysis cell to oxidize Breslow intermediates. After electrochemical oxidation, the reaction of the intermediate Nacylated thiazolium cation with primary amines is completed by passage through a heating cell to achieve high conversion in a single pass. The flow mixing regimen circumvented the issue of competing imine formation between the aldehyde and amine substrates, which otherwise prevented formation of the desired product. High yields (71−99%), productivities (up to 2.6 g/h), and current efficiencies (65−91%) were realized for 19 amides.
Read More
Org. Process Res. Dev., 2015, 19, 1424–1427 Robert A. Green, Richard C. D. Brown, and Derek Pletcher
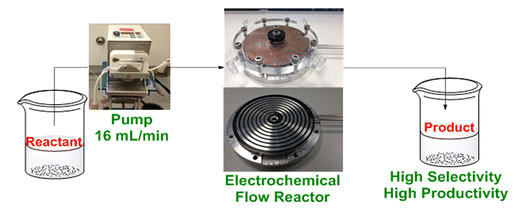
A Microflow Electrolysis Cell for Laboratory Synthesis on the Multigram Scale
A large microflow electrolysis cell for laboratory synthesis on a multigram scale is described. It is based on two circular electrodes with a diameter of 149 mm and a spiral electrolyte flow channel 2000 mm long, 5 mm wide, and 0.5 mm interelectrode gap. Using the methoxylation of N-formylpyrrolidine as a model reaction, it is demonstrated that the cell approaches 100% conversion in a single pass, and it is possible to achieve a reaction selectivity >95% and a product formation rate of >20 g h–1
Read More
J. Flow Chem. 2015 Robert A. Green, Richard C. D. Brown and Derek Pletcher
Understanding the Performance of a Microfluidic Electrolysis Cell for Routine Organic Synthesis
In order for microflow electrolysis cells to make their full contribution to routine laboratory organic synthesis, they must be capable of carrying out reactions with good selectivity and high conversion at a high rate of conversion. In addition to appropriate choice of the electrolysis medium and control of the overall cell chemistry, both the design of the electrolysis cell (including materials of construction) and the correct selection of the cell current and flow rate of the solution are critical in determining performance. The conclusions are tested using the methoxylation of N-formylpyrrolidine as the test reaction in a microflow electrolysis cell with a single, long, patterned flow channel.
Read More
Org. Lett., 2015, 17, 3290-3293 Robert A. Green, Derek Pletcher, Stuart G. Leach and Richard C. D. Brown
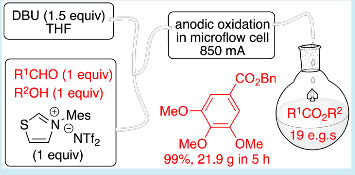
N‐Heterocyclic Carbene-Mediated Oxidative Electrosynthesis of Esters in a Microflow Cell
An efficient N-heterocyclic carbene (NHC)-mediated oxidative esterification of aldehydes has been achieved in an undivided microfluidic electrolysis cell at ambient temperature. Productivities of up to 4.3 g h−1 in a single pass are demonstrated, with excellent yields and conversions for 19 examples presented. Notably, the oxidative acylation reactions were shown to proceed with a 1:1 stoichiometry of aldehyde and alcohol (for primary alcohols), with remarkably short residence times in the electrolysis cell (<13 s), and without added electrolyte.
READ MORE
J. Flow Chem. 2014, 4, 2-11 Kevin Watts, Alastair Baker, Thomas Wirth
Electrochemical Synthesis in Microreactors
Different electrochemical microreactors for continuous flow synthesis are described in this review. Advantages of flow over batch type chemistry are highlighted as well as novel developments in construction of such devices.
Read More
ChemSusChem, 2012, 5, 326-331 Hill-Cousins, Joseph T., Kuleshova, Jekaterina, Green, Robert A., Birkin, Peter R., Pletcher, Derek, Underwood, Toby J., Leach, Stuart G. and Brown, Richard C.D.
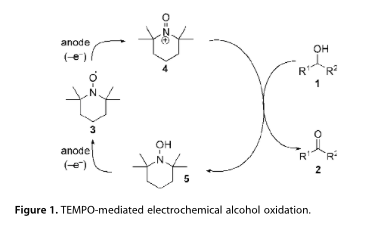
TEMPO-mediated electrooxidation of primary and secondary alcohols in a microfluidic electrolytic cell
A general procedure for the 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-mediated electrooxidation of primary and secondary alcohols modified for application in a microfluidic electrolytic cell is described. The electrocatalytic system utilises a buffered aqueous tert-butanol reaction medium, which operates effectively without the requirement for additional electrolyte, providing a mild protocol for the oxidation of alcohols to aldehydes and ketones at ambient temperature on a laboratory scale. Optimisation of the process is discussed along with the oxidation of 15 representative alcohols.